Human Biorepository
Unit info
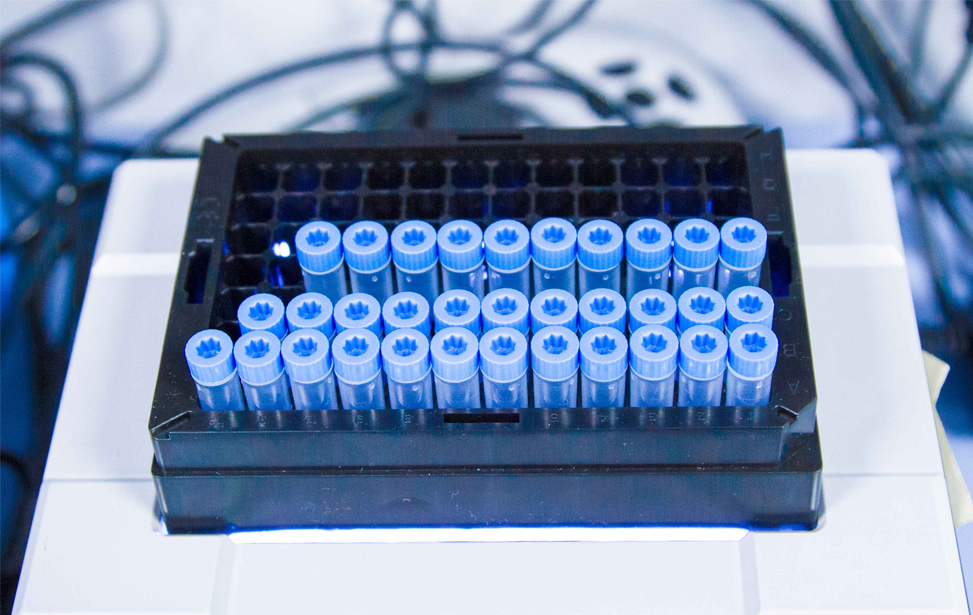
The IFOM Biorepository is a purpose-built core facility managing the collection, processing, storage and retrieval of human biospecimens from consenting donors (eg: DNA, RNA, blood, plasma, serum, urine, stools, tissue, cell lines, tissue blocks and slides, as well as patient-derived xenografts and patient-derived organoids), which has been created particularly to meet the increasing demand of investigators wishing to pursue translational or clinical cancer research studies, and to translate knowledge from basic research into therapies. The high-quality biospecimens and clinical data are a key resource in order to develop/validate new biomarkers and therapeutics.
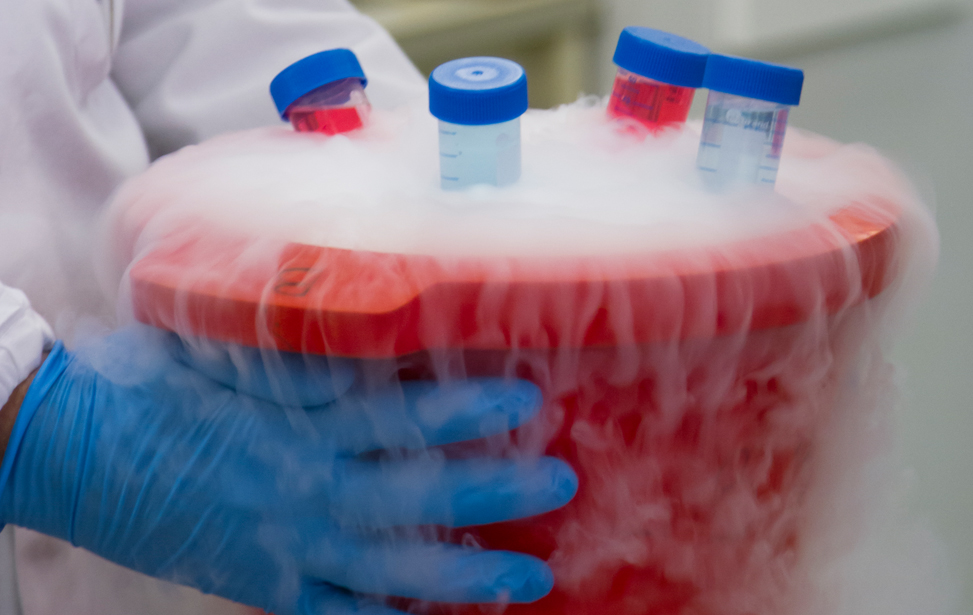
The dedicated platform consists of a cryogenic storage space fitted with O2 sensors and alarms for safety operations and containing two liquid nitrogen tanks where samples are stored in the vapour phase, and of separate space containing two +4°C fridges, two -20°C and two -80°C freezers (all connected to a back-up electrical generator in case of power outage), and a cabinet for the storage of paraffin-embedded tissue block and glass slides. Tubes, vials, etc, are labeled using 2D Data-Matrix barcode. Alarm sensors control liquid nitrogen level in each cryogenic unit and the temperature of mechanical freezers and refrigerators, and are connected to a centralized monitoring and recording system. Access to the sites is restricted to authorized operators through the use of individually assigned electronic badges.
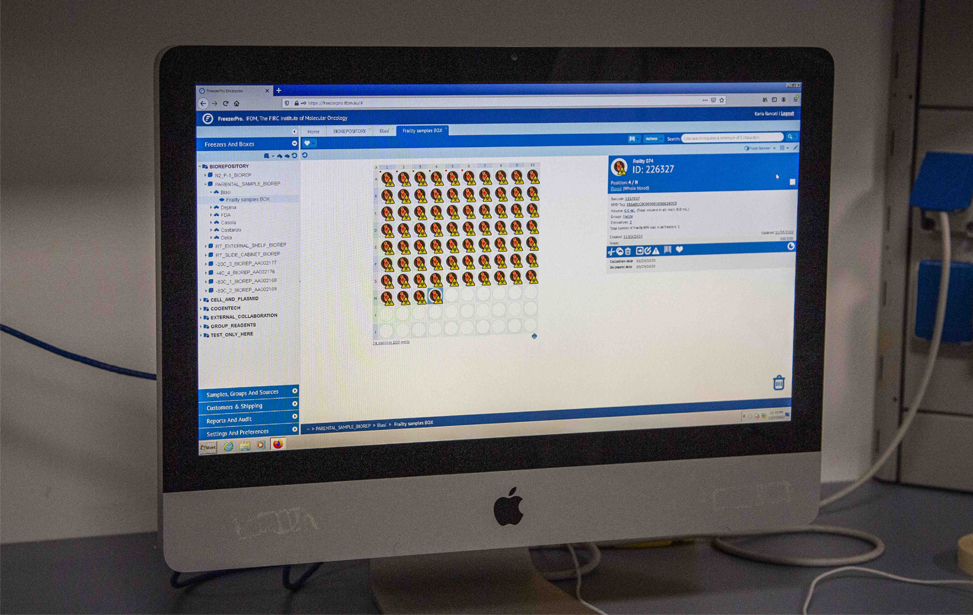
Complete sample inventory, management and workflow processes at the IFOM Biorepository rely on the use of web-based HIPAA-compliant RURO FreezerPro Elite software which provides sample tracking in and out of freezers, barcode printing and plate scanners support, user authentication and permission-based access restriction to samples, freezers and other elements, full audit trails.
Standard operating procedures (SOPs), handling of disaster recovery, security, protection and privacy of sample records are implemented as set in the IARC guidelines “Common Minimum Technical Standards and Protocols for Biobanks Dedicated to Cancer” Research and ISBER best practices for repositories. Only de-identified or anonymized human specimens and associated documents are stored in the biorepository.
The IFOM Biorepository is governed by an executive committee, which through a management committee and a specimens and data access committee, serves to oversee activities and best practices, compliance with current legislations, improve workflows.

Domenico Delia
Obtained the master degree in Biology in 1974 and a Specialty in Marine Biology at the University of Messina. From 1975 to 1981 undertook post-doctoral studies at the ICRF, London (now Cancer Research UK), investigating the biology of pediatric acute lymphoblastic leukemias and of the normal hematopoiesis process, allowing the development of immunological tools to diagnose and classify leukemias, and cell surface markers for the identification and purification bone marrow hematopoietic stem cells. Late 1981 joined the National Cancer Institute, Milano, carrying out research on non-Hodgkin’s lymphomas and underlying apoptotic resistance mechanisms. Elucidated the biochemical events leading to the ATM-dependent Chk2 activation in response to DNA damage. Established in vitro model systems of neurodegeneration in Ataxia Telangiectasia employing patient-derived iPSCs. Joined the IFOM in 2016 as collaborator, supervising the human iPSC lab and the Biorepository.
Human Biorepository Instruments
Unit members
Ilaria Rancati
Isabella Ponzanelli
Lab support
Cinzia Cancellieri
Laura Carmignani
Eleonora Verga
Stefania Lavore
Giuseppe Ossolengo